Ozone can be generated by exciting a flow of oxygen with sufficient electrical or optical energy. This will cause a certain amount of oxygen atoms to split and recombine with other O2 molecules nearby. This can be represented by the equation:
![]()
Under the typical treatment conditions, using a relatively pure oxygen stream and a corona discharge chamber, which utilizes a high voltage electrical arc, this reaction can produce up to 9 – 12 wt% ozone, although typically output is in 1 – 9 wt% ozone. The remainder of the stream is left as oxygen. The concentration is limited to this range because of the following reaction.
![]()
As ozone concentrations rise above this concentration, the destruction reaction (7) becomes more frequent, thus returning greater quantities to O2 and maintaining this equilibrium. This instability is also the reason ozone cannot be stored and must be generated immediately prior to application.
Due to its extreme instability and high oxidation potential, ozone is very powerful and indiscriminate in terms of reactivity with other chemical species. Ozone has been shown as an effective treatment for destruction of volatile organic compounds (VOCs), removal of metals, total suspended solids, organic carbon and many, many more as well as significant reductions to COD. In freshwater the half-life of ozone is typically ten to twenty minutes, whereas in wastewater, ozone has been documented as being entirely consumed within 8.6 seconds. This is due to the extreme amount of potential reactants that are present in wastewater, foremost of which for the purpose of this paper, is hydrogen sulfide. The simple structure of hydrogen sulfide makes it a very easy target for oxidation by ozone.
In addition to its high oxidation potential, ozone’s unique structure tends to create free radicals, which are chemical species that have unbonded electrons making them highly reactive, especially in water. Not only is the benefit of ozone’s direct reaction with various chemical species realized, but as part of these reactions additional free radicals can form, which can be even more reactive than ozone. Additionally, radicals tend to create additional radicals as they react, in what is termed a free radical chain reaction. These additional reactions are referred to as the indirect effects of ozone.
With the source of ozone generation being ambient air, it is the ultimate in sustainable and green chemical treatment. The current technology for producing ozone has benefitted from in excess of forty-five years of ongoing development, resulting in cost-effective and robust operation. Utilizing little more than an oxygen separator, a corona discharge chamber and some compressors and other electrical components, onsite generation of ozone is relatively simple and safe today. This is in sharp contrast to the majority of other treatments that are currently commercially available.
Due to the way ozone is produced, oxygen is necessarily going to be part of the treatment gas cocktail when using ozone. This is beneficial as oxygen is also an oxidizer. With an oxidation potential of 1.23 V, oxygen is a slower reactant than ozone, but an excellent complement. Aside from its ability to assist in oxidation, its primary benefit is increasing the DO concentration of the wastewater, encouraging the growth of aerobic bacteria, which do not create compounds that are odorous, corrosive or otherwise harmful to collection systems. It also eliminates the ability of SRB to produce sulfides, either by removing the SRB entirely or promoting the growth of aerobic species over the top that will oxidize any sulfides before they are able to enter the wastewater stream [EPA, 1985].

Picture 1: Anue Water Technologies Mobile Diagnostic Unit (MDU)
COMBINED USE OF OXYGEN AND OZONE FOR TREATMENT
In terms of a robust and green methodology for the treatment and prevention of odor and corrosion in collections systems, the combined forces of oxygen and ozone are at the top of the list. Oxygen is widely available, making up roughly 21 percent of the atmosphere, and has already been seen, is easily converted to ozone. The generation and infusion of these two gases into wastewater collection systems has proven to be a clean, safe and cost effective treatment. The first method of action is the powerful destructive effects of ozone on hydrogen sulfide, quickly converting it to sulfites and sulfates on contact. In addition, ozone’s antimicrobial properties can help to actually reduce the presence of SRB and other microorganism present on pipe walls. As a product of its reaction, oxygen is generated. This in turn adds more power to the oxygen portion of the treatment gas cocktail, which is providing secondary treatment by significantly increasing DO, and allows for more complete utilization of infused treatment gases. Oxygen will also oxidize hydrogen sulfide, but at a much slower rate than ozone.
Because of these indiscriminate and powerful oxidizing characteristics, the concern is sometimes raised regarding the possibility of ozone attacking the wastewater infrastructure itself. This is very unlikely to occur in application, especially in wastewater where liquid phase infusion is implemented. This is due to the 1) high ratio of liquid volume compared to pipe surface area per unit pipe length and 2) the extreme availability of reactants in the liquid portion. It is highly unlikely that ozone will survive long enough to affect the wastewater infrastructure.
TREATMENT AND TESTING METHODOLOGY
Anue Water Technologies (AWT) of San Diego, CA designs and manufactures a comprehensive range of systems, referred to as FORSe 5™, that utilize oxygen and ozone for effectively inducing clean, sustainable and cost effective treatment of odor and corrosion in collection systems. The direct treatment of the wastewater is achieved through a proprietary process of treating a factional flow of wastewater and returning this treated flow into the untreated flow, thus inducing change. This process is termed hydrodynamic infusion.

Picture 2: Tapped Force Main with Attached Infuser
EXPERIMENTAL CONDITIONS
The testing was conducted at a municipal waste water district in southern Arizona that had received numerous complaints from an upscale neighborhood regarding the presence of strong sewage odor. The odor was determined to be originating from the collection system by way of manholes located in their neighborhood. Traditionally, sodium hypochlorite (bleach) had been used for odor control, essentially system wide. This treatment, however, had not proven to be effective in controlling the odor being generated within the collection system. This is evident both by the complaints that were made as well as through the collection of H2S vapor concentration data.
AWT has a trailer with a self contained treatment system onboard that was used in this test. It is referred to as the Mobile Diagnostic Unit (MDU) and shown in picture 1. The MDU was placed at the treatment site and attached to a 2-inch tapping saddle on the force main (picture 2) on August 18, 2011. The goal was the elimination of odor at the problematic downstream manholes, as well as total and dissolved sulfides, and to increase DO to levels above aerobic, or 1 mg/L. Three downstream manholes were utilized for sample collection, and are referred to as MH1, MH2 and MH3, with 1 being the closest to the point of treatment. MH1 was approximately 2 miles downstream from the treatment site and MH2 and 3 were an additional ¼ to ½ mile from MH1.
AWT began collection of grab samples, prior to treatment, at MH1 and placed an OdaLog sensor on August 17, 2011 at approximately 10:30 am. Grab samples were analyzed for pH, temperature and DO with a Hach HQ40d, H2S via Drager tubes and total and dissolved sulfides were measured with LaMotte Sulfide test. Additionally, a third party sampling service was hired to collect liquid grab samples and OdaLog data from MH1 and solely OdaLog data from MH2 and 3, starting August 18 at approximately 9:30 am. Third party grab samples were analyzed for pH, temperature, ORP with an Oakton pH11 Series, DO with a Hach SensION 6, and sulfides were again measured with LaMotte Sulfide test.
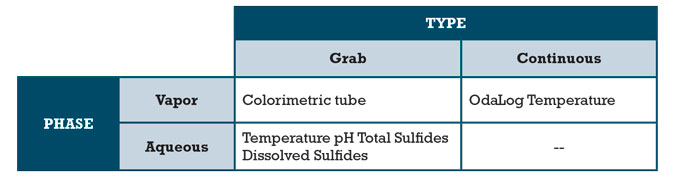
Table 2 – Sample Phase and Type Matrix
A LOOK AHEAD
Treatment at the force main was initiated midday on August 18 and initially consisted of liquid phase infusion of 40 liters per minute O2 on a continuous, twenty-four hour basis and infusion of 120 grams per hour O3 whenever the force main pumps cycled on. On Friday August 19 the injection amounts were decreased to 20 liters per minute O2 and 60 grams per hour O3. The force main was 8-inch diameter and had a flow of 15,000 GPD, resulting in a significantly long retention time of twenty-five hours, between the treatment site and MH1. The bleach that had been used for odor control was ceased on August 16 at approximately 9:30 am, giving the system approximately forty-eight hours to resume untreated conditions. In next month’s installment, we will take a closer look at the test results. ◆
Anue Water Technologies is a privately owned, U.S. company dedicated to providing innovative solutions for improving the processing and treatment of water and wastewater. Anue serves the global market with a network of sales representatives and service providers to actively support applications development, leading to optimized solutions—working with municipalities, wastewater operating and industrial companies who encounter challenges with wastewater, to create tailored, highly effective solutions for treating odor and corrosion. For more information, visit www.anuewater.com.
____________________________________________
MODERN PUMPING TODAY, August 2017
Did you enjoy this article?
Subscribe to the FREE Digital Edition of Modern Pumping Today Magazine!
![]()