In part 1 of this series, we discussed the corrosion issues associated with hydrogen sulfide in wastewater collection systems. The main classes of reactions used for control of hydrogen sulfide include oxidation, sulfide scavengers (iron salts), pH adjustment, alternate oxygen source/sulfate substitute, and the combined use of oxygen and ozone for treatment. We will briefly describe these processes in this month’s and next issue’s contributions to this series.
OXIDATION
Chemical oxidation of hydrogen sulfide is accomplished through use of a compound with a high oxidation potential, called an oxidant, such as hydrogen peroxide or sodium hypochlorite (bleach). This method of treatment involves the direct oxidation of H2S into more benign forms, such as sulfites, sulfates, and elemental sulfur. However, it does not affect the presence of SRB, which will likely continue to grow over time. Upon cessation of treatment, expression of hydrogen sulfide can be even worse than before, due to an increased output of the growing SRB layer. In addition, it is likely that increasing quantities of treatment chemical will be required over time for the same reason.
SULFIDE SCAVENGERS (IRON SALTS)
Chemicals that interact with hydrogen sulfide and sequester, or scavenge, the sulfur into a relatively insoluble form, such as ferric chloride and ferrous chloride, can be used to remove sulfur from the cycle entirely. This treatment has no effect of the presence or production of the SRB layer, and cessation of use will see a return to the emission of hydrogen sulfide, possibly at an elevated level.
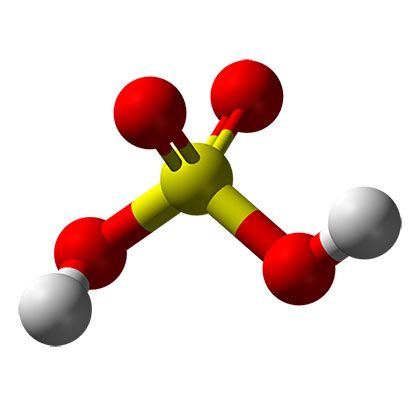
For this reason it is likely that increased usage of time will be experienced as well. These chemicals will aggressively remove DO from the wastewater, can form scales on iron surfaces, such as pipes and pumps, and become an ongoing source of sedimentation due to the reaction of iron with sulfur forming FeS precipitate within the collection system. Continued and/or elevated risk of corrosion is a significant challenge with the use of ferrous and ferric chloride, due to the generation of acids as part of the scavenging reaction. An example of a reaction in which hydrochloric acid is generated is shown in equation (5).
![]()
The bulk scale addition of iron to the collection system also presents the hazard of contamination of downstream solids. In fact, CERCLA considers iron salts a persistent environmental hazard. This aspect can significantly add to plant and biosolids processing costs.
pH ADJUSTMENT
Due to the manner in which its ions dissociate in the aqueous phase, the release of hydrogen sulfide from wastewater will not occur if the pH is at 9 or above. Through use of compounds that can induce and maintain significant increases to pH, such as sodium hydroxide or magnesium hydroxide, it is possible to take advantage of this trait and “trap” the sulfide in solution. This can be an expensive process, due to the volume of treatment chemical required to maintain such an elevated pH level and the relatively higher expense per gallon (liter). Also, treatment chemicals can be difficult to keep in solution, as is the case with magnesium hydroxide, especially in colder weather. A good illustration of this is magnesium hydroxide, which has a solubility of only 0.012 g/L in water; bordering on the line of insolubility. There is again no treatment or reduction to SRB, which can cause the problem to grow over time. Also, once pH decreases below 9, as can happen downstream of treatment and/or at the treatment plant, hydrogen sulfide is readily released, possibly at an increased rate due to the increased solution concentration.
ALTERNATE OXYGEN SOURCE/SULFATE SUBSTITUTE
In an anaerobic environment, the microbiology in the collection system will utilize oxygen from a nitrate (NO3) molecule more readily than from a sulfate (SO4) and as a result benign nitrogen is released rather than hydrogen sulfide. Chemicals like calcium or sodium nitrate are commercially available and can be utilized for this purpose. These chemicals are expensive and they actually feed and grow the SRB layer, potentially requiring higher volume for treatment over time. Upon cessation of treatment, the expression of hydrogen sulfide can be even worse than before. Excess wet well build-up requiring increased clean-out cycles due to the addition of the waxes used to stabilize the nitrate molecules can be encountered downstream in the collection system. In addition, emerging federal and state regulations are beginning to include nitrate concentrations on discharge limitations. There is seldom much real time, active monitoring of wastewater sulfide levels, so enough chemical to control peak H2S values is typically added on a constant basis. By treating for peak values with chemicals such as these, there is a very high likelihood that excess nitrate will be present and actively added to the wastewater, requiring additional denitrification processes or fines, both of which can be very expensive.
An issue with all chemicals is that in order to introduce them into a collection system, a bulk quantity must be stored nearby. In order to ensure that there will always be chemicals available for treatment, there must be continued deliveries to the bulk storage tank. To make sure that the environment is not adversely affected (directly), various engineered controls, such as secondary containment and leak monitoring, must be designed, implemented, and maintained. Essentially, money is being poured down the drain on a recurrent basis with no real solution to the problem ever taking place.
Ideally, successful treatment of wastewater odor and corrosion would cease sulfide production, quickly eliminate sulfides that are present, present no additional hazard to life or the environment, do no harm to the collection system itself, and create no additional challenges downstream. In addition it must be cost effective. Such a solution is becoming available through the novel approach of introducing ozone and oxygen into collection systems as a means of odor and corrosion control.
Ozone has long been used in water treatment, dating back to at least the late nineteenth century, primarily for disinfection and polishing of drinking water. In Europe, ozone treatment of water is a very common process. It is well established that ozone’s superior environmental sustainability and relative safety versus chemical systems has established it as the favored current and future technology. The controlled use of ozone as a treatment does not produce any harmful byproducts that could contaminate or harm the environment or ecology. Typically, the only by products from its reaction is O2 and inert oxides. In recent years, interest in its use in the wastewater industry has led to the development of new and sustainable (green) technology for the treatment of odor and corrosion in wastewater collection systems.
Ozone is a special, naturally occurring form of atmospheric oxygen. Instead of two oxygen atoms it has three, represented by its chemical formula O3. This third oxygen atom makes it a highly reactive molecule with a very high oxidation potential. In fact, it has the highest oxidation potential of any commercially available molecule and fourth highest overall with an oxidation potential of 2.07 V. Above it, in terms of oxidation potential, are atomic fluorine (F•, 2.87 V), the hydroxyl radical (•OH, 2.86 V), and atomic oxygen (O•, 2.42 V).
A LOOK AHEAD
In next month’s installment, we will take a closer look at the combined use of oxygen and ozone for treatment as well as set the stage for the treatment and testing methodology. ◆
Anue Water Technologies is a privately owned, U.S. company dedicated to providing innovative solutions for improving the processing and treatment of water and wastewater. Anue serves the global market with a network of sales representatives and service providers to actively support applications development, leading to optimized solutions—working with municipalities, wastewater operating and industrial companies who encounter challenges with wastewater, to create tailored, highly effective solutions for treating odor and corrosion. For more information, visit www.anuewater.com.
____________________________________________
MODERN PUMPING TODAY, July 2017
Did you enjoy this article?
Subscribe to the FREE Digital Edition of Modern Pumping Today Magazine!
![]()